TouchMD a product of Nextech is pleased to offer AmSpa Informed Consents and Intake forms to those that have purchased these packages. Proof of purchase is required to activate AmSpa Informed Consents & Intake Forms. For a complete listing of all consents and intakes available, please see the AmSpa Informed Consent and Intake Library section below.
Purchase Instructions
- Log on to the AmSpa portal at https://americanmedspa.org/medspaforms with your AmSpa member login
- Select “Store”
- Under the “Categories” section, select “Forms and Consents”
- Purchase the desired package * NOTE: TouchMD only supports the English-based consent and intake forms.
Activation Instructions
- Log on to the AmSpa portal at https://americanmedspa.org/medspaforms with your AmSpa member login
- Locate your Proof of Purchase
- Send your Proof of Purchase to [email protected]
- TouchMD Support will send a confirmation within 24 hours that the resources have been activated in your TouchMD consent library
Adding/Editing AmSpa Informed Consents and Inatke Forms
- Log onto the TouchMD Dashboard (dashboard.touchmd.com) with your TouchMD Username and Password

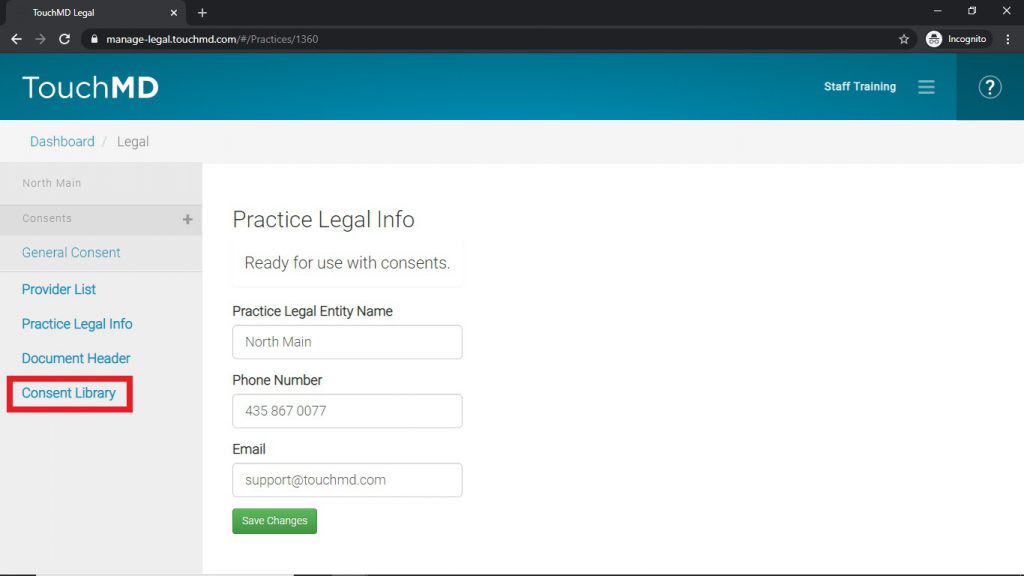
- Select “Legal”

- Select “Consent Library”
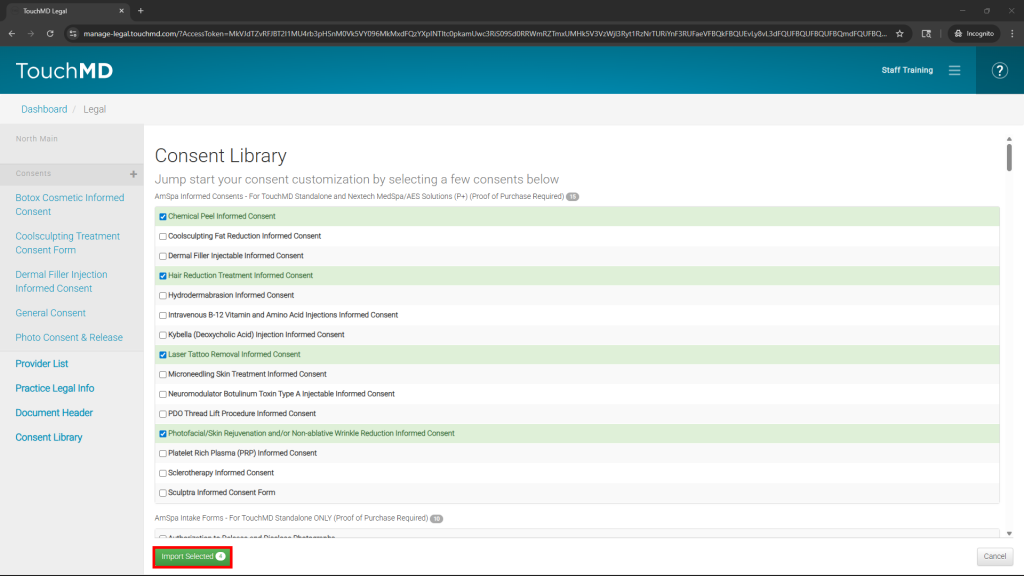
- Select the desired consents and “Import Selected”
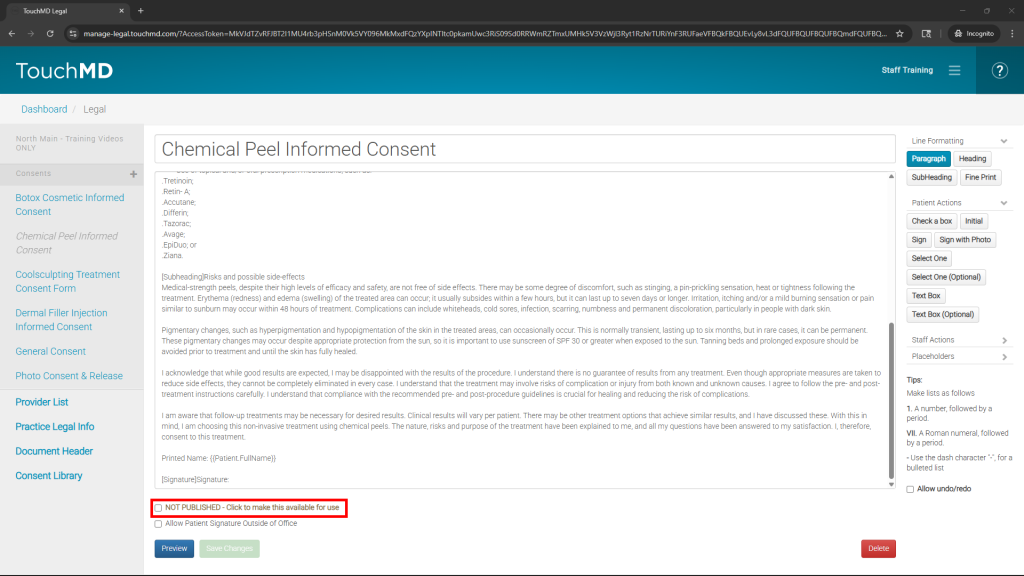
- Review and modify the imported consent to reflect your practice policies
- Preview your consent to view and verify all of your consent changes by selecting “Preview” (Optional)
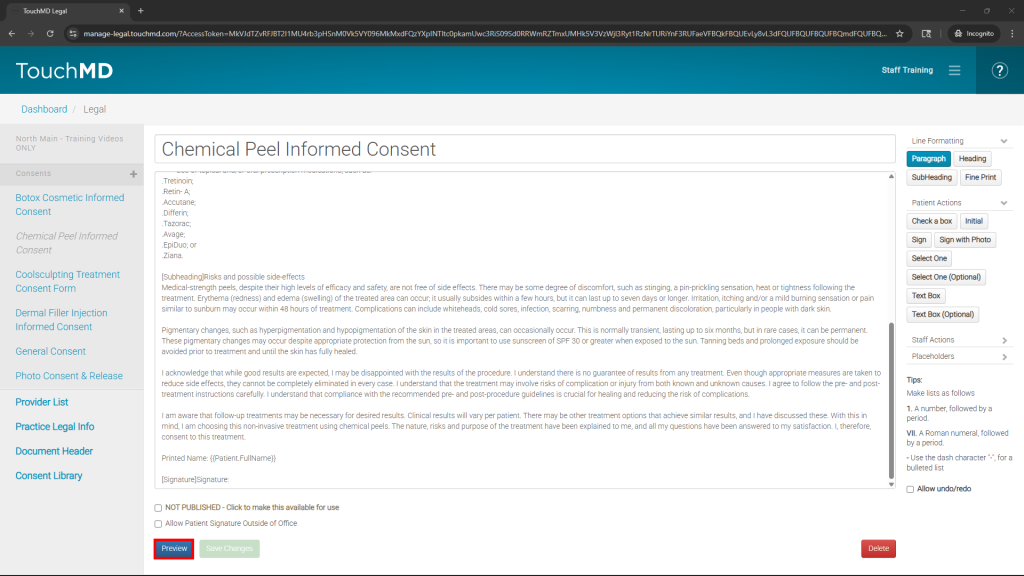
- Publish the consent for use with patients by checking the box next to “NOT PUBLISHED – Click to make this available for use”
Using the AmSpa Informed Consents and Intake Forms
Please review the following help pages to learn how to use your AmSpa Informed Consents and Intake Forms in TouchMD:
AmSpa Informed Consents and Intake Forms Library
Informed Consents
- Chemical Peel Informed Consent
- Coolsculpting Fat Reduction Informed Consent
- Dermal Filler Injectable Informed Consent
- Hair Reduction Treatment Informed Consent
- Hydrodermabrasion Informed Consent
- Intravenous B-12 Vitamin and Amino Acid Injections Informed Consent
- Kybella (Deoxycholic Acid) Injection Informed Consent
- Laser Tattoo Removal Informed Consent
- Microneedling Skin Treatment Informed Consent
- Neuromodulator Botulinum Toxin Type A Injectable Informed Consent
- PDO Thread Lift Procedure Informed Consent
- Photofacial/Skin Rejuvenation and/or Non-ablative Wrinkle Reduction Informed Consent
- Platelet Rich Plasma (PRP) Informed Consent
- Sclerotherapy Informed Consent
- Sculptra Informed Consent Form
Intake Forms
- Authorization to Release and Disclose Photographs
- INFORMED CONSENT – COVID-19 PANDEMIC
- Patient Acknowledgment of Provider
- Patient Consent for Photography
- Patient Consent to Telemedicine Services
- Patient Consent to Telemedicine Services – COVID-19
- Patient Consent to Treatment
- Patient Contact Authorization
- Patient Financial Responsibility Agreement
- Notice of Privacy Practices